
All matter is made of atoms. Atoms consist of a central nucleus with electrons circling round it, like the planets circling the Sun. Chemical reactions involve only the electrons circling the nucleus; nuclear reactions involve the nucleus itself.
There are three types of nuclear reaction: nuclear fission, nuclear fusion, and radioactivity. This Page is about radioactivity, separate Pages deal with nuclear fission and nuclear fusion.
This Page assumes you are familiar with the structure of the nucleus, particularly with the meaning of nucleon number and isotope.This Page is designed to be read through in order, but you can also link to each Section by clicking on its name in the index. The owl at the end of each Section brings you back to this point.
An atomic nucleus containing only protons would be unstable and very quickly disintegrate because of the repulsive electrostatic force between the protons. The neutrons in the nucleus provide a binding force. Only certain arrangements of protons and neutrons are stable. In other arrangements the binding force is not strong enough and the nucleus is unstable and will eventually disintegrate. Atoms with unstable nuclei are said to be radioactive. The disintegration of the nucleus of a radioactive atom is called radioactive decay, or sometimes a radioactive transformation, and all the various particles produced by a radioactive decay are called the decay products.
All elements except fluorine exist in the form of two or more isotopes. When we are talking about isotopes we must always give the nucleon number. For some elements such as radon all the isotopes are radioactive, but for most elements some of the isotopes are radioactive and some are stable. Fluorine exists only in the form of fluorine-19, which is not radioactive.
A word that is used in atomic physics is nuclide. A nuclide is an atom with a specific atomic number and nucleon number, for example magnesium-24. A radioactive isotope is correctly called a radionuclide, although radioisotope is also very commonly used.
Remember that it is nuclides (isotopes) that are radioactive not elements. For example, it is incorrect to say that potassium is radioactive, because only one of its isotopes, potassium-40, is. Only if all the isotopes of an element are radioactive, for example radon, can we refer to it as a radioactive element.

We measure the activity of a radioactive source in becquerels, after Antoine Becquerel (1852 - 1908) who discovered radioactivity. One becquerel (Bq) is a mean rate of one radionuclide decay per second.
When we think of radioactivity we usually think of Marie Curie (1867 - 1934) and her husband Pierre (1859 - 1906). Together with Becquerel they laid the foundations of nuclear physics. After the death of her husband and Becquerel Marie continued working on radioactivity by herself. She is the only person to have been awarded two Nobel Prizes for science, one for physics and one for chemistry. The original unit of radioactivity was the curie (Ci), named after her, but this was inconveniently large and was later replaced by the becquerel. One Ci was the activity of 1 g of radium-226 and is 3.7 × 1010 Bq.
We can measure radioactivity with a Geiger-Muller Tube, or Geiger Counter. This is described on another Page.
The stability of a radionuclide is expressed in terms of its half-life. If a radionuclide has a half-life of ten hours it means that in any ten hour period half of the radionuclides in any sample will have decayed - whatever the size of the sample.
Different radionuclides have different half-lives. Half-lives for radionuclides vary from 2 × 1015 years for neodymium-144 (this is a hundred thousand times the age of the Universe!) to 125 nanoseconds for astatine-213 (a nanosecond is a thousand millionth of a second!), and everything in between.
Remember that a radioactive source can, and often will, contain several different radionuclides, so although we can talk about the radioactivity of a source, half-life refers only to a particular radionuclide.
Half-life is discussed in greater detail, with some maths and some worked examples, on the Half-life Page.
Alpha particles are very strongly ionizing (this is explained on another Page) and because they are charged they are slightly deflected (their path is bent) by electrical and magnetic fields. As they travel through the air (or anything else) they leave behind a trail of ionized molecules, and this uses up their energy, so they have a range of only a few centimetres in air and are stopped by a thick sheet of paper, even the outer (dead) layer of the skin. They travel at about 10% of the speed of light. Once they have lost their energy they eventually pick up two free electrons and become ordinary atoms of helium.
Beta particles are only weakly ionizing. This means that as they travel through the air, or anything else, they do not leave behind as many ionized molecules as alpha particles, so lose their energy less quickly and travel further. Like alpha particles they are deflected by electrical and magnetic fields, but because they have only about 1/8000 the mass of an alpha particle they are deflected far more - but in the opposite direction of course because they carry an opposite charge. They travel at about 90% of the speed of light. They have a range of a few metres in air, and are stopped by aluminium or other metal foil. Once they have lost their energy they become ordinary free electrons - they might even become the electrons which turn a beta particle into a helium atom!
Gamma radiation does not leave ionization trails so it does not lose energy travelling through air, and as it has no charge it is not deflected by electrical or magnetic fields. It travels at the speed of light. Gamma rays have a very short wavelength, a few picometres, shorter than the distance between the molecules in the air. This means that gamma rays (photons) can pass through the gaps between the air molecules for long distances before they hit one. Gamma radiation can therefore travel several kilometres in air, but shorter distances in solids and liquids where the molecules are closer together. They are stopped by several centimetres of lead. Gamma rays are not themselves ionising, but when a gamma photon does eventually hit a molecule it may cause atoms in that molecule to emit ionizing particles.
This site also contains a vast amount of useful and interesting information about every known element, including details of all their isotopes.
Thorium-234 is itself radioactive and is a beta emitter. As the nucleus decays a neutron becomes a proton and an electron, and the electron is ejected at very high speed - this is the beta particle. Remember that the beta particle comes from the nucleus of the atom, not from the electrons in the shells round it. Here is a symbolic equation for the formation of a proton and an electron from a neutron.
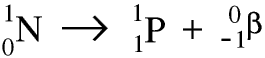
Whenever a nuclide loses a beta particle the nucleon number stays the same but the atomic number goes up by 1.
Proactinium-234 is itself a beta emitter, and emits a beta particle to become uranium-234.
Uranium-234 is itself radioactive.... We are now into a radioactivity series: a radioactivity series goes on until eventually a stable nuclide is produced. The series starting with uranium-238 ends, fourteen transformations later, with a stable isotope of lead. It may be interesting to see the whole series (but no one will ever expect you to remember it!) - here it is.

The Solar System, that is, the Sun and all the planets including the Earth, was formed about four thousand six hundred million years ago. All the Earth's uranium-238, uranium-235, thorium-232, and also potassium-40 (half-life 1.3 × 109 years) and rubidium-87 (half life 47.5× 109 years) were present in the Earth at the time it was formed. Almost all other naturally occurring radionuclides (except carbon-14) in the Earth are the decay products of uranium-238, uranium-235 and thorium-232. We sometimes refer to nuclides formed by radioactive decay as daughter nuclides or progeny.
Carbon-14 is produced by the action of cosmic rays on nitrogen in the atmosphere and is discussed later. There are also a number of Man-made radionuclides: these are produced in nuclear reactors or in nuclear research laboratories and are discussed more fully on the Page on nuclear fission.
Almost all radionuclides are either alpha or beta emitters; some but not all alpha and beta emitters are also gamma emitters; only a very few radionuclides are pure gamma emitters, with no alpha or beta emission.
A coin balanced on its edge is unstable because its centre of gravity is higher than it would be if the coin were flat on the table, that is, it possesses more gravitational potential energy. Similarly a radionuclide is radioactive because it possesses more binding energy and so is in a higher energy state than one which is stable. When it decays its energy state is reduced, and the extra energy has to go somewhere. Energy is transferred to the alpha or beta particle produced by the decay; if there is still some energy left over this is emitted as a photon of gamma radiation. A gamma photon has a nucleon number and atomic number of zero and no electrical charge. A rearrangement of the protons and neutrons in the nucleus is usually associated with gamma emission.
Radioactive decay also produces heat.
Here are the first three stages of the uranium-238 radioactivity series in the form of symbolic equations.
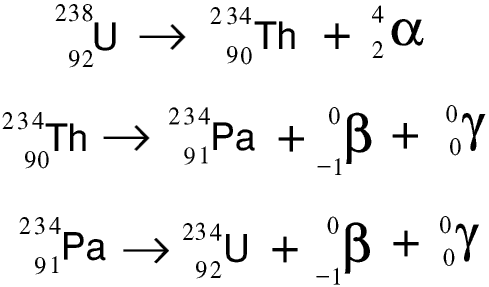
Even if we could start with a quantity of pure uranium-238, as it decayed the decay products would build up around it, and very quickly it would not be pure.... As these decay products are themselves radioactive, with shorter half-lives than uranium-238, the radioactivity of a radioactive source containing uranium-238 increases rather than decreases with time. Marie Curie noticed this and started to analyse the decay products: this lead to her discovery of radium and polonium.
Uranium is needed for nuclear power stations and many other purposes. It is mined in several parts of the World. After the uranium has been extracted from the uranium ore the tailings (material left behind) contain all the decay products and so are highly radioactive
.
There are ninety two naturally occurring elements in the Earth, and almost all of them have at least one radionuclide. We are therefore surrounded by radioactive substances, in the rocks of the Earth's crust, in the water in the seas, rivers and lakes and which we drink, in the materials our homes are made of, in the air we breathe and the food we eat. Even our bodies are radioactive: a normal adult is a radioactive source with an activity of about 4000 Bq.
The Earth also receives cosmic radiation from space. The radiation from the radioactive substances around us and from space make up the background radiation. The amount of background radiation varies from place to place on the Earth's surface. In all experiments involving measuring radioactivity we must remember to measure the background radiation before we begin and subtract it from our measurements. We also receive ionizing radiation from X-rays and other medical procedures and from certain other sources.
The radioactive dose which we receive from any source of radiation is measured in sieverts (Sv), or more usually millisieverts. This is more fully described on the Page on Safety and Health. Most people in the British Isles receive a radiation dose of about 2.5 mSv a year, made up from all these sources. This is more fully discussed in the Page on health and safety
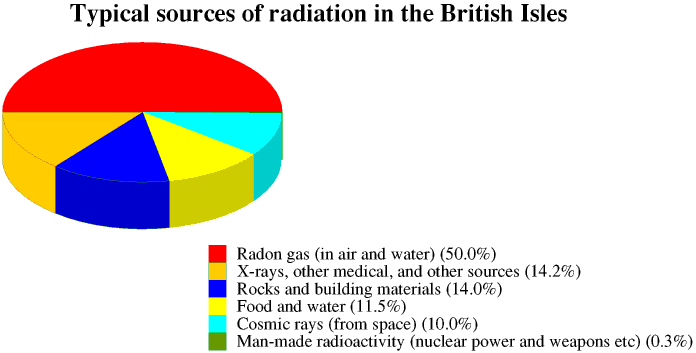
In some parts of Britain, particularly around Edinburgh, Aberdeen and Cornwall, background radiation levels are higher because the underlying rocks are mainly granite, and many buildings are built of granite: granite contains radioactive substances which decay to form radon gas. Radon collects in cellars and underfloor spaces, and so good ventilation of houses and other buildings is very important.
Radon is also brought to the surface in the water from springs heated by radioactivity deep in the Earth's crust: in Finland the dose from background radiation can reach 7 mSv a year; in some parts of the world it can be higher still.
Most cosmic rays are absorbed by the Earth's atmosphere, particularly the ozone layer. At high altitudes you are above most of the atmosphere and so radiation levels are much higher. Pilots and other aircrew, frequent fliers and astronauts therefore receive very much higher doses than most other people. The destruction of the ozone layer will lead to higher radiation levels even at low altitudes - this is discussed in the Page on Atmospheric Pollution - to link to it please click hereThe Earth has always been radioactive and all living things have always been subject to radiation. Low levels of radiation are harmless, in fact there is evidence to show that low levels of radiation are actually essential to life, and that evolution is driven by radiation - this is discussed later.
Since the 1940s there has been a very tiny increase in background radiation levels due to Man's activities: nuclear research programmes, nuclear power stations and nuclear weapons. The atomic bombs dropped on Hiroshima and Nagasaki, and nuclear accidents such as Chernobyl, were and are still personal tragedies for hundreds of thousands of people, but the effect on the Earth as a whole, and on most human, plant, animal and other forms of life, has been and remains insignificant. Mankind and all other living things on this planet are far more threatened by global warming, acid rain, the destruction of the ozone layer, deforestation, and the pollution of the land, rivers and seas by heavy metals such as lead, cadmium and mercury, pesticide residues, PCBs, dioxins and other industrial chemicals.

Helium (2He) is the second most common element in the universe because it was formed in the immediate aftermath of the Big Bang. The visible matter in the Universe is about 76% hydrogen and 23% helium. Helium is also produced by nuclear fusion deep inside stars including our Sun. It is light enough to escape from the Earth's atmosphere, and so exists on the Earth only because it is being continually produced by radioactive decay: every alpha particle eventually ends up as an atom of helium-4. On the Earth it exists mainly as helium-4, with one part in a million of helium-3 and even smaller amounts of heavier isotopes such as helium-5 and helium-6 - these are radioactive. Helium boils at minus 269oC so liquid helium is used for keeping things very cold. It is also used for filling balloons and airships: to link to a Page about this please click here ![]()

Carbon (6C) is present in all living things - this is discussed more fully in the Page on the Carbon Cycle - to link to it please click here ![]()
There are two main isotopes: carbon-12 makes up about 99% and carbon-13 about 1%.There is also a third isotope, carbon-14, which is radioactive. Carbon-14 is formed by the action of the neutrons which form part of the cosmic rays from space interacting with nitrogen atoms in the atmosphere. A nitrogen-14 atom gains a neutron and loses a proton to form carbon-14.
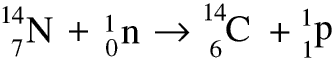
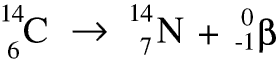

Potassium (19K) is the 8th most common element in the Earth. It is present in the Earth's core, mantle and crust, in the seas, and in all living things except some bacteria. It consists of about 93% potassium-39, 7% potassium-41 and 0.012% potassium-40. Potassium-40 is radioactive, with a half-life of about 1.3 × 109 years. This of course means that your body is radioactive! Very unusually, potassium-40 decays in two different ways. About 89% of potassium-40 atoms undergo a normal beta decay to form calcium-40, the nucleon number staying constant and the atomic number going up by one. Calcium-40 is not radioactive. But about 11% of potassium-40 atoms decay by a process called electron capture: the nucleus captures an electron, which then combines with a proton to form a neutron. The nucleon number stays the same, but the atomic number goes down by one, so potassium-40 becomes argon-40. Argon-40 is not radioactive and makes up about 1% of the atmosphere.
Most of the heat generated in the Earth's core and mantle comes from the radioactive decay of potassium-40; the heat produced in the mantle in this way causes the convection currents which move the tectonic plates of the Earth's crust. In living things its radioactivity is considered to be the main cause of genetic variation and therefore the driving force of evolution.
Potassium-40 can also be used for dating things. This is discussed on the Page on radioactive dating - to link to it please click hereMan needs to obtain all the different nutrients, proteins, fats, carbohydrates, vitamins and minerals etc, he requires from the foods that he eats. There are hundreds of different proteins but they are all made up of about twenty amino-acids. Not all proteins contain all the amino-acids Man needs, but meat and fish and other animal products such as eggs and cheese are very good sources of most of them. Some people however choose not to make any use of any animal products, not even milk (or even wool). These people are called vegans. To get enough of all the essential amino-acids they need vegans need to eat more, and a far greater variety of, fruits and vegetables than people who eat meat and milk products. As a result vegans have a diet which is very rich in potassium, and the excess potassium is excreted in the urine. This sort of diet is perfectly healthy, but it does mean that the urine of vegans contains high levels of potassium and so is slightly more radioactive than the cooling water from nuclear power stations!


Isotopes of radon (86Rn) are produced by the decay of uranium-238, uranium-235 and thorium-232. There are 31 isotopes and all are radioactive. Radon-222 is the most stable, with a half-life of 3.8 days. Radon is present in the atmosphere, in rocks such as granite, and in water, particularly where water heated by radioactivity deep in the Earth’s crust comes to the surface. It accounts for about 50% of the background radiation.
All isotopes of radon are alpha emitters. As dangerous as the gas itself, which is not absorbed into the bloodstream in the lungs, are the solid radioactive decay products such as lead-210, with a half-life of twenty two years, which can adhere to dust particles and enter the body in food or water this way.
Radon is used as a tracer - this is discussed further on another Page.

The thorium reactor is discussed on the Page on nuclear fission. It has the great advantage over uranium reactors that it does not produce any materials that can be used in nuclear weapons.

Uranium (92U) is needed by Man for nuclear power stations, nuclear weapons and for nuclear research programmes. Uranium and thorium are the only radioactive substances that are needed by Man in significant quantities; these are obtained by extracting them from the Earth’s crust by mining: almost all the other radionuclides needed by Man are obtained from the decay products of the naturally occurring isotopes of uranium and thorium, and from nuclear power stations and research centres. Both uranium-235 and uranium-238 are radioactive, but uranium-235 is also fissile: when a uranium-235 nucleus is struck by a neutron it splits in two, making two new nuclides and releasing more neutrons. Nuclear fission produces far more energy than radioactive decay, and this can be harnessed in nuclear power stations and atomic weapons. Nuclear fission is much more fully discussed on its own Page.
When a uranium-235 nucleus is split by being struck by a neutron more neutrons are produced. If any of these neutrons strike other uranium-235 nuclei they also will be split releasing more neutrons: this is a chain reaction. Nuclear weapons and nuclear power stations involve chain reactions of this sort.
In Nature today uranium is about 99.3% uranium-238 and 0.7% uranium-235. Uranium-238 is not fissile but it does absorb neutrons. The presence of such a high percentage of neutron-absorbing uranium-238 mixed with the uranium-235 reduces the probability that the neutrons released by the fission of one uranium-235 atom will hit and cause the fission of another and so sustain the chain reaction. So for many purposes, both civil and military, the uranium must be enriched, to increase the percentage of uranium-235 in it. There is just one problem with this: for every kilogram of enriched uranium produced we end up with several kilograms of depleted uranium, that is, uranium with less uranium-235. Depleted uranium is also produced when the spent fuel rods from nuclear power stations are reprocessed.
Unfortunately depleted uranium is absolutely useless, but it is still highly radioactive, and also all uranium compounds are poisonous.
What's the cheapest substance in the world?
Baked beans? They're only 8p a tin down the Market.
No, cheaper than that.
How about water?
No, cheaper than water.
Cheaper than water? Air, then.
No, cheaper than air.
I give up. What's cheaper than air?
Your rubbish!
I'm rubbish? Be like that then.
No, your rubbish. Rubbish is so cheap you can't even give it away, you have to pay someone to take it away!
It’s true of course: rubbish and waste products are the cheapest things in the world, and the more dangerous the waste product the cheaper it is, that is, the more it costs to have it someone to take it away.
In warfare, artillery shells and missiles designed to destroy tanks and other very heavily armoured targets must be as heavy as possible. We need to fill them with something really dense. The ideal material would be platinum, with a density of 21.5 kg/dm3, although gold, with a density of 19.3 kg/dm3, would be almost as good. But the military do not use these: they use depleted uranium, with a density of 19.0 kg/dm3, because, whatever the dangers of scattering over the countryside a dust which is both highly toxic and remains radioactive for millions of years, it is very, very cheap.