An uncharged atom contains as many electrons in shells round the nucleus as there are protons in the nucleus. Electrons have a negative charge and protons an identical positive charge, so the atom itself has no charge. If the electron shell now gains or loses an electron the atom becomes charged: we call a charged atom an ion. Groups of atoms joined together, for example acid radicals such as nitrates or sulphates, can also exist as ions. Molecules are bound together by chemical bonds formed by the arrangement of the electrons in the shells round the nuclei of the atoms in the molecule: if this is changed by ionization the bonds may be broken and the way in which the atoms are joined together in the molecule may be altered. Ionization may be produced by ionizing radiation. Ionizing radiation is potentially dangerous to all living things because it may change the structure of the complex organic molecules inside living cells.
We can measure the activity of a radioactive source with a Geiger-Muller Tube, or Geiger Counter.

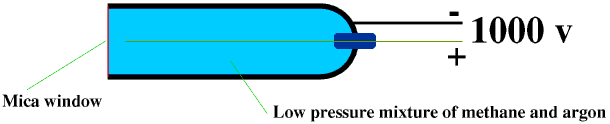
A Geiger-Muller Tube consists of a metal tube with a hemispherical end, and a mica window. The tube is filled with low-pressure gas, usually a mixture of methane and argon. A thin metal wire, the anode (+), runs down the middle of the tube, and this is surrounded by a cylindrical wire mesh, the cathode (-). The anode is kept at 1000 V above the cathode. Any ionizing radiation entering the tube through the window will ionize the gas in the tube, and the ionized particles will be attracted to the electrodes, causing a burst of current to flow between them. Once the alpha or beta particle or gamma photon has given up all its energy there will be no more ionization and so no more current until another one enters the tube. Each burst of current is therefore the result of one alpha or beta particle or gamma photon entering the tube. Each burst is counted. We often give the radioactivity of a source in counts per minute. Sometimes (particularly in films!) a Geiger-Muller Tube is made to produce an audible “click” each time it detects a burst of current.
We can make the path followed by ionizing radiation visible by using a cloud chamber. The radiation leaves behind a trail of ionized gas, and the ions act as nuclei start the condensation. This is more fully discussed in the Page on The Three States of Water: to read it please click here
The chemical properties of an element depend upon the arrangement of the electrons in the electron shells round the nucleus, and this arrangement depends only on the number of protons in the nucleus, not the number of nucleons. So all isotopes of an element are chemically identical. (This makes separating them difficult; there is more about this on the Page on nuclear fission.) But the way a radionuclide decays depends only upon the arrangement of the nucleons in the nucleus, so does not depend upon how, or whether, it is chemically combined with other elements. For example, carbon-14 is radioactive and will decay whether it is in the form of pure carbon, or an atom in a compound such as carbon dioxide, or glucose, or in a protein, or in any other compound containing carbon. But it makes no difference at all to mice and elephants and human beings whether the proteins in their bodies contain carbon-12 or carbon-14.
Carbon-14 is a beta emitter and loses an electron to become nitrogen-14. But nitrogen has a different arrangement of electrons in the shells around the nucleus, so the electron bonds that hold the molecule together will be broken and the molecule will be changed - the effects are therefore identical to ionization.
To put this into perspective, an adult human body contains about 16 kg of carbon, of which about 0.0000000016 g is carbon-14 - and carbon-14 has a half-life of 5730 years!

We are surrounded by radioactive substances and other sources of ionizing radiation and inevitably we absorb some of the radiation which reaches us. The amount of radiation our body absorbs is measured in grays (Gy), after the English radiobiologist L H Gray (1905 - 1965). (No relation to me.)
However, because different sorts of ionizing radiation have different effects on our bodies we need to measure not just the amount of radiation we are receiving but also its effect. The effect of all the ionizing radiation we receive, from all sources not only radioactivity, what is called our equivalent radiation “dose”, is measured in sieverts (Sv) or more usually millisieverts (mSv), after the Swedish physicist Rolf Sievert (1896 - 1966).
To help understand the radiation units becquerel, gray and sievert, think of a rainstorm. The becquerel measures the amount of rain that is falling, the gray measures the amount of rain that falls on us, and the sievert measures how wet we get. For more about this you can visit the GCSE Nuclear Radiation Web site - to do so please click here ![]()
Background radiation is all around us and has always been; tests on very simple organisms such as bacteria have shown that low doses of radiation are probably actually essential for all living things. In the past the Earth was much more radioactive than it is today and over hundreds of millions of years organisms have evolved ways of repairing small amounts of radiation damage. Doses of less than 100 mSv a year, far higher than the background level in most places in the world, are usually considered to be harmless. Background radiation is discussed at greater length in the next Section.
Moderate doses of ionizing radiation may damage cells. A damaged cell may behave in an abnormal way; one example of abnormal cell behaviour is cancer. For reasons not yet fully understood the cells associated with the production of blood cells (particularly in the liver, bone-marrow, lymph nodes and spleen) are very much more sensitive to ionizing radiation than other cells, which is why leukaemia is often linked with radiation.
Cells that have been changed are sometimes called mutant cells. But you cannot pass the effects of radiation onto your children unless the cells that will eventually become eggs or sperms, in the reproductive organs, have been changed. Then a mutation may be produced. Very tiny and usually quite harmless mutations may be produced by natural radiation - this is discussed in the next Section. But higher doses of radiation may lead to undesirable mutations and abnormal babies.
Very high doses of ionizing radiation may kill cells; this leads to radiation sickness. In mild cases this may involve skin sores, hair loss, diarrhoea and vomiting; it is a sad fact of life that almost all we know about severe radiation sickness comes from studying the victims of nuclear weapons such as the Hiroshima and Nagasaki atom bombs. For this reason radiation sickess is more fully discussed in the Page on nuclear fission.
Outside the body alpha emitters are not dangerous because alpha particles are stopped by the skin. But if alpha emitters are taken into the body, from gases or dusts in the air, in drinking water or in or on food, or through cuts or other injuries, they are very dangerous indeed, because the range of an alpha particle is very short and it will do very great damage to a very small area. Beta and gamma radiation are relatively less harmful because they travel further and so may pass through or out of the body.
Radiation can kill cells and in medicine controlled doses of radiation can be used to treat cancers. Radionuclides can also be used in the body as tracers. The medical uses of radioactivity are discussed on another Page.
Radionuclides are often used in science and medical laboratories and other places. For some tasks you must use an open source. These are potentially very dangerous because of the possibility that radioactive material might be released into the atmosphere or soil or water, and to handle open sources you need a very special licence: if you hold such a licence you will already know a lot about radioactivity and will probably not be reading this Page (except perhaps as a critic - please e-mail me with your comments). In schools you may be allowed to use a closed (or sealed) source. Here the radioactive source is permanently sealed into a labelled thick metal container with a small plastic window at one end to let the radiation out. You must always handle the source with special tongs and keep it at arms length, and never point it at yourself or anyone else. When it is not in use it is kept in another labelled locked metal box in a labelled locked metal cupboard in a labelled locked room which is used only for storage.
