When we talk about gas we usually mean the gas which is piped into our houses and which we use for heating and cooking. This is mainly a gas called methane. Methane has no smell so we usually add a harmless smelly substance to it so that we know if we have left a gas tap on or there is a gas leak. Methane comes from gas fields deep under the Earth's surface so it is often called natural gas. Natural gas, and also coal gas which was piped into our homes before the discovery of the natural gas fields under the North Sea, is discussed in another page on my Web Site - to link to it please click here ![]()
There are however lots of other gases, such as oxygen, nitrogen, hydrogen, helium, chlorine, butane, propane and carbon dioxide, and of course air itself is a mixture of many different gases. Many of these gases are used in our factories, hospitals, schools and homes but they are not usually piped into them as natural gas is, they must be transported and stored in other ways. The problem is that at ordinary atmospheric temperature and pressure all gases have very low densities (a kilogram of hydrogen would fill a space 3 m by 2 m by 2 m) and it would be quite impractical to transport and store them as gases like this. We need to reduce their volume by increasing their density, and this can be done by turning them into liquids, or compressing them, or both.
For the rest of this page we are taking normal room temperature to be about 20oC and normal atmospheric to be about 1 bar (1000 mb). So 5 bars is five times normal atmospheric pressure. Units of pressure are further discussed in another page in this web site - to visit it please click hereThe boiling point of many gases depends upon the pressure: the higher the pressure the higher the boiling point. For example the boiling point of water is 100oC at the standard atmospheric pressure of 1013 mb, but inside a pressure cooker the pressure is about 2 bars and the boiling point of water is about 120oC, while on the top of Mount Everest the pressure is only about 260 mb and the boiling point of water is about 69oC.
Similarly the boiling point of butane is about 0.5oC at atmospheric pressure, but at 2 bars it is about 20oC. So if we compress it to 2 bars it will be a liquid at all temperatures below 20oC.
Butane and many gases with boiling points of less than room temperature can be stored as liquids at room temperature by compressing them enough to raise their boiling point to the temperature of the room. Inside the container the gas is at its boiling point. The pressure inside is the pressure needed to make the boiling point of the liquid equal to the temperature of the room. If the temperature of the room then goes up slightly some of the liquid in the container turns into a gas and this raises the pressure and so also the boiling point, similarly if the temperature goes down some of the gas turns into a liquid and lowers the pressure and so also the boiling point. The pressure in the container is therefore not constant but is always the pressure needed to make the boiling point of the gas the same as the temperature of the room. As the pressure of the gas in the cylinder depends only upon the temperature of the room and not on the amount of liquid in the cylinder only a simple pressure regulator is needed.
Gases which can be stored as liquids under pressure in this way are propane and butane (sold under trade names such as Calor Gas and Camping Gaz), sulphur dioxide and ammonia and many others. (The very special case of carbon dioxide is discussed later.)
Usually these gases are stored in refillable cylinders with valves - the cylinders are very heavy and often cost more than the gas in them. At atmospheric pressure propane has a much lower boiling point than butane (see below in this Section), so at 20oC the pressure inside a propane cylinder is about 5 bars, but that inside a butane cylinder is only about 2 bars. So small quantities of butane for recreational use (Camping Gaz etc) are often sold in light-weight disposable cartridges. For the safe handling of these cartridges see the section on Safety at the end of this page. We can also store some other gases, such as sulphur dioxide, in light-weight disposable cartridges, but not propane as the pressure is much higher so the container needs to be much stronger and would not be light-weight!
Cylinders containing inflammable gases are usually fitted with connectors with left-hand threads, where to tighten the nut you have to turn it anti-clockwise. This is intended to make it impossible to connect a cylinder containing an inflammable gas to a pipe intended to carry oxygen or air or any non-inflammable gas.
A butane cylinder contains both liquid and gas. When we open the valve we want only gas to come out: we must therefore always use the cylinder in an upright position, and also always let it stand for a few minutes after moving it, to allow the liquid to settle, before using it. If any liquid were to pass through the valve with the gas, when it reached the burner it would flare up in a highly dangerous way.
The boiling point of butane at atmospheric pressure is minus 0.5oC, which means that when the temperature is below freezing point the pressure in a butane cylinder is atmospheric and when you turn on the tap nothing will come out. Even at slightly higher temperatures the pressure is very low so butane cannot be used out of doors in the winter. For this reason most building and other industrial sites, and also hot-air balloonists, use propane not butane - the boiling point of propane at atmospheric pressure is minus 42oC, and even at -25oC the pressure in the cylinder is more than 2 bars.
LPG (Liquefied petroleum gas) is the generic name for commercial butane and propane. To find out more about LPG please visit the LPG web site ![]()
LNG is Liquid Natural Gas - this is discussed in the page on Natural Gas.

Oxygen, nitrogen and many other gases have what is called a critical temperature. Above its critical temperature a gas cannot be liquefied by pressure alone. The critical temperature for butane is 152oC so below this temperature it can be liquefied just by compressing it. But the critical temperature for oxygen is minus 118oC, which means that above this temperature we cannot liquify it just by compressing it, no matter how much hard we try. All the gases in the air (except carbon dioxide which is discussed later), and also hydrogen, methane and ethane and many other gases have critical temperatures below room temperature.
We can store these gases in cylinders at high pressure, sometimes up to several hundred times atmospheric pressure - the higher the pressure the greater the amount we can store in the cylinder. As we use up the gas the pressure in the cylinder falls so we need a very complex pressure regulator to maintain a constant rate of flow out of the cylinder. The cylinders must be very strong and so are very heavy and expensive: they cost a lot more than the gas inside them!
Some gases that are often stored in this way areHybrid solutions are not feasible. For example the critical temperature for ozone is about -12oC so you could store ozone as a liquid under pressure in a freezer. The problem is, what happens if there is a power cut and the freezer goes off?

Almost all gases can be liquefied even at atmospheric pressure by cooling them enough - the boiling point of oxygen at atmospheric pressure is minus 182oC. We can therefore store and transport oxygen as a liquid at atmospheric pressure. In order to minimise losses from evaporation we must keep it cold in a special flask similar to the vacuum (Thermos) flask used to keep drinks hot. This special flask is usually called a Dewar Flask after Sir James Dewar (1842 - 1923) who invented it. He was doing a lot of work on liquid gases - he was the first person to liquify hydrogen - and originally invented the Dewar Flask to keep liquid gases cold; it was only later that his invention was developed to keep hot things hot.
We almost always store a gas with a critical temperature above room temperature as a compressed liquid; for a gas with a critical temperature below room temperature we have the choice of storing it as a compressed gas in a cylinder or as a cold liquid in a Dewar. Usually we use cylinders of compressed gases for storing relatively small amounts, for example in school science laboratories and small workshops; only very big users would use liquid gases.
Liquid nitrogen (minus 196oC) is very cheap because it is a by-product of the production of liquid oxygen so is used for keeping things very cold; liquid helium (minus 269oC or 4 K) is used in some very low temperature experiments.
To read more about the gases present in the atmosphere, their preparation and their uses, please click here ![]() To read about temperature scales please click here
To read about temperature scales please click here ![]()

For many substances, including water and carbon dioxide, increasing the pressure raises the boiling point and lowers the freezing point, while reducing the pressure lowers the boiling point and raises the freezing point. At atmospheric pressure water boils at 100oC and freezes at 0oC, but if we increase the pressure we raise the boiling point and lower the freezing point. This is how a pressure cooker works and why we can make snowballs - for more about this please click here ![]()
Similarly if we lower the pressure we raise the freezing point and lower the boiling point. If we reduce the pressure enough we may be able to lower the boiling point and raise the freezing point until they are the same: this is the triple point. If we lower the pressure further still we may even be able to raise the freezing point and lower the boiling point until the freezing point is above the boiling point! For water the triple point is 0.01oC and 6 mb. At this pressure the freezing point and boiling point of water are the same, but the triple point pressure is so low, less than one hundredth of atmospheric pressure, that this is of no practical importance to most of us.
However, the triple point for carbon dioxide is minus 56.6oC and 5.11 bars, and this is of considerable practical importance because atmospheric pressure is below the triple point pressure. This means that at atmospheric pressure the freezing point of carbon dioxide is higher than its boiling point, and liquid carbon dioxide cannot exist. So if we cool carbon dioxide gas at atmospheric pressure it turns into not a liquid but a solid. The freezing point of carbon dioxide at atmospheric pressure is minus 78oC. Solid carbon dioxide is called Dry Ice because, unlike ordinary ice, it does not melt to leave a liquid. Dry Ice is often used for keeping food, particularly icecream, cold. It has the additional advantage that as it evaporates it produces a gas which is heavier than air and does not support life so the food is surrounded by a gas which helps preserve it. Dry Ice is also used for many other purposes, including special effects in the theatre and at concerts.
Dry Ice is very cold and you must never handle it except with special tongs or while wearing special gloves - ordinary gloves are not good enough.
(If the atmosphere is very dry the dew point may be below 0oC and so water vapour may condense as crystals of ice not droplets of water, but this has nothing whatever to do with the triple point. This is discussed in the page on the three states of water - to link to it click here ![]() )
)
If we compress carbon dioxide to above the triple point pressure of 5.11 bars the boiling point becomes higher than the freezing point so liquid carbon dioxide can exist. The pressure needed to raise the boiling point of carbon dioxide to room temperature is about 27 bars, so cylinders of carbon dioxide contain liquid carbon dioxide at a pressure of about 27 bars.
If we want carbon dioxide gas, for example to pressurise fizzy drinks or to put out a fire, we use a cylinder in which gas is taken from the top of the cylinder, as in the drawing
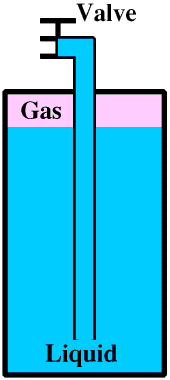

All stored gases (even compressed air!) are potentially dangerous, for a number of reasons.
Any gas stored under pressure is dangerous because of the possibility of the container rupturing (splitting open). If this were to happen jagged pieces of metal (shrapnel) would be violently projected in every direction. The danger of rupturing is greatly increased if the cylinder is heated, for example in a shed in strong sunlight (or of course in the event of a fire), because of the increase in the pressure of the gas in the container.
Some gases, such as sulphur dioxide or chlorine, are harmful or poisonous, and any release of such a gas, whether suddenly by a cylinder bursting or slowly by a faulty valve, is potentially dangerous.
If oxygen escapes it will raise the oxygen level in the air in the area, and this will create a very serious fire hazard - a spark in a photocopier could set fire to all the plastic components inside it. It is not generally realised that the proportion of oxygen in the air (21%) is very close to the safe limit: at 25% wet green plants will burn, so a single bolt of lightning could wipe out a whole forest even in the rainy season.
If an inflammable gas is escaping from a broken pipe or leaking cylinder and burning as it does so it might be very frightening but it is not usually dangerous unless it sets fire to nearby objects. The danger with inflammable gases is primarily explosion, where the gas has first escaped and mixed with the air and then the mixture has been ignited, for example by a spark. Gases such as natural gas (methane) are lighter than air and disperse quite quickly so the danger does not last long once the leak has been stopped, but butane and propane are heavier than air and can collect in low places (drains, cellars etc). Boats such as cabin cruisers are particularly at risk, because even the tiny amount of gas released in the time between turning on the cooker gas tap and lighting the flame can sink into the bilges and accumulate there. Then after a few months the build-up is such that the tiniest spark can cause an explosion which will tear the boat apart. A boat blowing up is not always the result of a bomb! It is therefore very important indeed that the bilges are cleaned out with a compressed air line at regular intervals.
All gases which are heavier than air are potentially suffocating and you must always have very good ventilation when working with them. You should never store or use them in a cellar. Safety with disposable butane cartridges is particularly important, because as the cartridge is put into the cooker or lamp a small hole is cut into it which cannot be resealed. If the cartridge is not put in properly it may fall out or leak and all the gas in it will escape - this is more than enough to suffocate everyone in a tent. Always change disposable cartridges in the open air, and always put gas lanterns outside a tent before going to sleep.
Gases which are stored as liquids at atmospheric pressure are very cold, and a gas which is stored as a compressed liquid at room temperature may become very cold if the container leaks or breaks because of the evaporation of the liquid. In either case very bad freezer burns, frostbite or worse may result.

© Barry Gray August 2010