Sunlight consists of a mixture of light of (almost) every wavelength from 740 to 390 nm. Different wavelengths are seen as different colours, but we can only actually see the individual colours if they are separated in some way.
Refraction and the spectrum
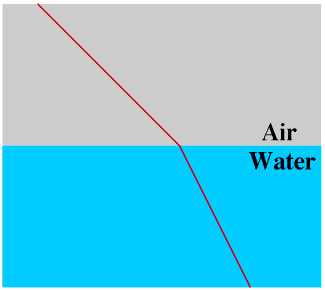
Light travels through a vacuum, and also through air, water, glass and many other materials (media). When light passes from one medium to another the rays may be bent, or refracted. This is why a stick may appear to be bent where it breaks the surface of a lake and why a swimming pool appears much shallower than it really is.
Some of the light is also reflected off the surface: this is why you can see yourself in a shop window. If the glass is thick enough you may actually see yourself twice, once where the light is reflected off the outside of the glass and once where it is reflected off the inside. Birds may fly into windows if they see reflections of trees or the sky in them. You can reduce the danger to birds by putting stickers onto windows where this often happens, ideally a red sticker of a bird of prey silhouette: you can buy packs of suitable stickers from the RSPB. This reflection of a part of the light is important when we come to consider the rainbow, at the end of this Page, but is not considered further until then.

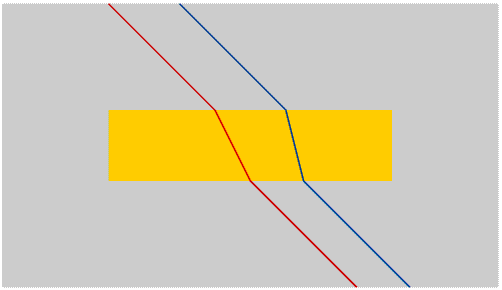
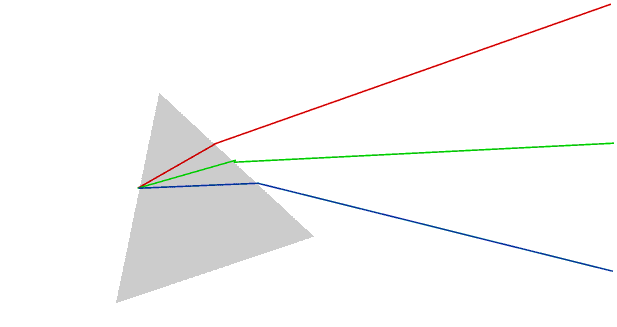

Once we have separated the colours we can isolate any colour by using a moveable slit.
We also often see colours in oil films or soap bubbles. This happens because the oil film is only a few nanometres thick. Some of the light is reflected off the top surface and some the bottom surface. Because the thickness of the oil film is about the same as the wavelength of the light the two reflected rays may interfere with each other. In a soap bubble, gravity is pulling the liquid downwards so the bubble is thinner at the top than the bottom, and also the water is evaporating, so the thickness of the soap film is constantly changing and so are the colours. The colours are not usually very clear, and may include browns and other non-spectral colours. Just before the bubble bursts part of the film has become so thin that it appears black: you can see this most clearly in big bubbles.

This bubble is in the course of construction. You can make bubbles more than 2m in diameter which last for several minutes with this special wand and a bucket of special bubble mixture - search on giant bubble making to find out how to buy them. Enjoy! - and send me some pix of the bubbles.
Of course only a very little light is reflected off a bubble in this way, just enough for us to be able to see it.

Very clear spectral colours are often produced by the recorded side of a compact disc, CD ROM or DVD - here the colours are produced by diffraction. But diffraction and interference are outside the scope of this web page.
If we heat a metal block it will start to radiate energy. The frequencies of the radiation it emits depend upon its temperature. Initially the frequencies of the radiation are less than that of light. This is infra-red - we cannot see infra-red radiation but we feel it as heat. As the block gets hotter it begins to emit visible light as well, first just red (red hot), then progressively the other spectral colours, until it appears to be white (white hot). Then it starts emitting even higher frequencies - these are ultra-violet. We cannot see ultra-violet radiation but it can damage our eyes and cause chemical changes in our skin.
We often refer to the colour of the light spectrum produced by a particular source in terms of its colour temperature: this is the temperature of a body that will produce a similar spectrum. For various reasons colour temperatures are usually given in Kelvin rather than degrees Celsius, but you can convert from Kelvin to degrees Celsius by subtracting 273, for example 273 K is 0oC. There is more about the Kelvin temperature scale on another Page of this Web Site - to link to it please click here html"> As described on this Page remember that temperatures are given in Kelvin not degrees Kelvin.
As described on this Page remember that temperatures are given in Kelvin not degrees Kelvin.
A candle flame is very yellow, with a colour temperature of about 1900 K; we define white light as having a colour temperature of 6500 K.
The atmosphere also absorbs some of the shorter wavelengths, so at midday the Sun itself appears slightly yellow, with a colour temperature of about 5000 K, while in the early morning or late evening it is much redder. Daylight is white light because it contains light from the whole sky not just the direct light from the Sun, and has a colour temperature of about 6500 K.
This is more fully discussed on the Blue Sky Page of my web site.
When atoms are excited, for example by heat or electricity, they may give off radiation. The frequency of this radiation depends only upon the type of atom (element). Most types of atom give off radiation of only one (or possibly two) wavelengths, and the atoms of many metallic elements give off radiation in the visible range. Sodium atoms for example give off a bright monochromatic yellow light. That is why if we are cooking vegetables on a gas cooker and the water boils over the flame goes yellow: the water contains salt and salt (sodium chloride) contains sodium atoms. It is also of course why sodium street lights are yellow. Other metals give off light of different colours, for example lithium compounds produce a deep red, copper green and potassium lilac (two spectral lines).
If we pass light from any source, say a burning airship or a distant star, through a spectrometer we can identify many of the substances present, in the flames or the star, just by looking at the spectral lines. This is how we can now be certain about the cause of the Hindenburg airship disaster - to link to the Web Page on this please click here ![]()
Atoms which radiate light of a particular frequency also absorb light of that frequence. In 1868 an English astronomer was using a spectrometer to analyse light from the Sun and noticed that one spectral line was missing. He concluded that this was because an element present in the Sun was absorbing light of this frequency. This element had never been found on the Earth, so he called it helium after Helios the Greek Sun God, but with the -ium ending because the frequency of its spectral line made him think it was a metal, like sodium, calcium, lithium, potassium etc. It is in fact a gas, and is the second commonest element in the Universe, having been created in the immediate aftermath of the Big Bang. It does exist in minute quantities in the Earth's atmosphere but it was not identified on the Earth until 1895. Once it was realised it was a gas they tried to change its name to helon, to go with argon, neon, xenon etc, but the name stuck. There is more about helium on another Page of this Web Site which deals with its use in balloons and airships - to link to it please click here ![]()
When we are considering light, or any other radiation, sometimes it is most helpful to think about the rays of light, travelling in straight lines. However sometimes it is more helpful if we think about the light waves spreading out in a circular pattern from the light source, like the ripples spreading out in circles on the surface of a pond when we drop a small stone into it.
If a light source is producing light of a certain frequency and is a certain distance from us and is not moving then the wave fronts it is emitting will reach us at the frequency of the light. If however the light source is moving towards us, in the time it takes one wave front to reach us the light source will have moved closer to us so the next wave front will have less far to travel and will arrive more quickly. This means that the time interval between successive wave fronts reaching us will be less so the light will appear to be a higher frequency. Similarly if the light source is moving away from us the light will appear to be a lower frequency.
This is called the Doppler Effect, and is why when a train goes through a station sounding its whistle the pitch of the sound changes as it passes us.
In astronomy the distant galaxies are moving away from us at very great speeds. This means that the light they are emitting appears to be a lower frequency, shifted towards the red end of the spectrum. By measuring the red shift we can work out the speed at which the galaxies are moving away from us.
The first European to publish an account of the rainbow was Sir Isaac Newton (1642 - 1727). Most people give him the credit for this explanation, but in fact almost everything Newton wrote in his Theory of Optics had been discovered six hundred years earlier, by the 11th century Arab mathematician al-Haytham. (Newton was familiar with his work.)
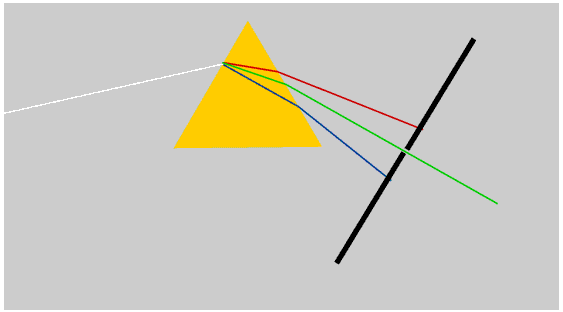
We see a rainbow when rays of sunlight that have been both reflected and refracted by raindrops enter the eye. This diagram shows only the rays which produce the rainbow.
Some books say that the rays are totally internally reflected inside the rainbow, but this is not true, and Newton himself did not say it. Only a part of the light is reflected: the angle at which the rays must strike the inside of the raindrop in order to reach the eye is not shallow enough for total internal reflection to take place.
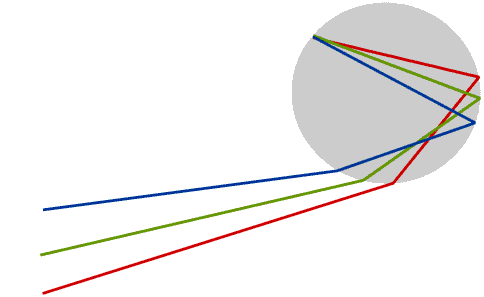
Only raindrops which are in exactly the right position produce coloured light rays which enter the eye: this means the Sun must usually be quite low in the sky. The raindrops which produce a rainbow lie in an arc of a circle, and the raindrops which produce the red are in a slightly different position to those which produce the violet - and of course are constantly changing.
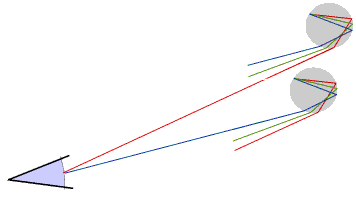
A primary rainbow has the red on the outside. Sometimes we may see a second, much fainter, rainbow with the violet on the outside: this is produced when rays of light that have been reflected inside each raindrop a second time enter the eye.
It is extremely difficult to take a good photograph of a rainbow, but to visit a site with some beautiful rainbow pictures please click hereSir Isaac Newton was one of the greatest scientists and mathematicians of all time, but there was also a very dark side to him. He was what is called a Magus and dabbled in alchemy and other strange beliefs. He believed that the rainbow had mystical properties and so had to have seven, the mystical number, of colours.
“Everyone knows” the seven colours of the rainbow: red, orange, yellow green, blue, indigo and violet. There is just one problem: if challenged to actually count the colours in a real rainbow the majority of people can only see six. Sir Isaac inserted indigo to bring the number up to seven. There is a colour called indigo, as any artist will tell you, but it is not present in the rainbow.
At one time Newton’s Discs were very popular, and if you are at school your school might have one, although they are not often used today. A Newton’s Disc consists of a disc painted with sectors of “all the seven colours of the rainbow.” When you spin it all the colours should combine to form white.
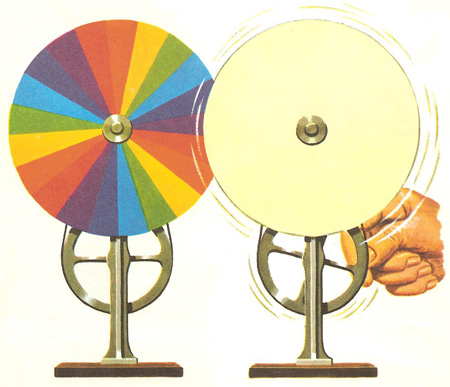
There are only three problems: most people see a muddy brown rather than white; you get a much better result by using a disc with just the three primary colours of red, green and blue (see Page on Colour Vision); and, be honest, have you ever seen these colours in a rainbow?
You are welcome to look for a distinct colour between blue and violet in a rainbow (or spectrum), and if you do find one please e-mail me to say you have done so. The only Rule is that you must be looking at a real rainbow or spectrum: because of the way coloured images are produced (this is discussed on the Page on Colour Vision referred to above) a photograph, drawing, television or computer image, computer print, poster or coloured picture in a book or anything similar will not do.
© Barry Gray August 2019